Hydrogen fuel cell catalyst performance is hard to assess without real-time insight into gas reactions. Gas analyzers offer a precise and efficient solution.

By monitoring CO, CO₂, CH₄, and other syngas components in real-time, gas analyzers help researchers evaluate catalyst activity and stability, accelerating hydrogen energy technology development.
Although the importance of gas analysis is established, its practical role in steam methane reforming (SMR) for hydrogen production demands deeper exploration.
Why Is Gas Analysis Essential for Evaluating Hydrogen Fuel Cell Catalysts?
Understanding a catalyst’s performance requires more than voltage or current measurements—it demands visibility into the chemical reality of the reaction.
In hydrogen fuel cells, especially during steam methane reforming (SMR), the core mechanism involves complex transformations of CH₄ and H₂O into CO, CO₂, and ultimately H₂. These transformations are governed by catalyst activity, selectivity, and stability—all of which are reflected in the composition and dynamics of the gas phase.
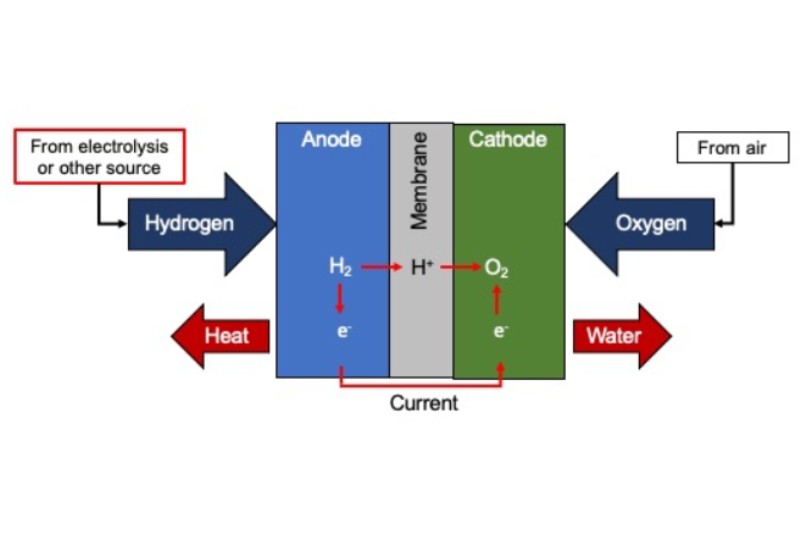
Basic Structure of Hydrogen Fuel Cells
The problem is that traditional catalyst evaluations often rely heavily on electrochemical testing or surface characterization, which provide limited insight into what actually happens during gas-phase reactions. For example, a catalyst might show good current density in a lab test, but still perform poorly in a real reforming system due to side reactions, incomplete conversion, or heat-induced degradation. Without knowing the exact ratios and fluctuations of key gases like CO, CO₂, and CH₄, such issues remain undetected.
This information gap becomes even more critical when considering catalyst degradation mechanisms. Catalysts can lose activity over time due to sintering, poisoning, or thermal stress. These phenomena often manifest not in immediate performance drops, but in subtle shifts in gas product ratios—like increasing CO/CO₂ or declining H₂ yield—long before any visual or structural signs of damage emerge. Gas analysis is often the only tool sensitive enough to detect these early indicators.
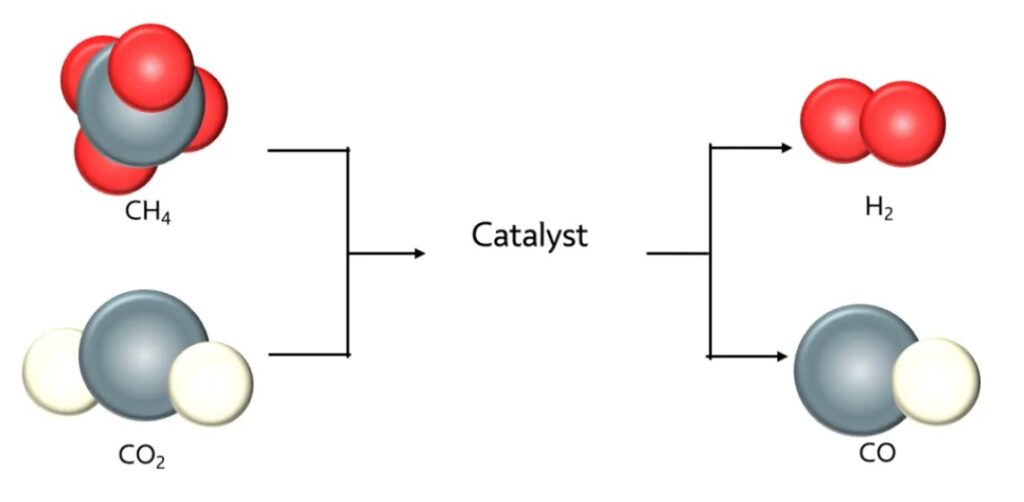
ESEGAS analyzers provide continuous, quantitative insight into these gas dynamics. By measuring key outputs at the reactor exhaust, researchers can evaluate how a catalyst behaves over time, under stress, and across varying temperatures and pressures. Such data is essential not only for identifying high-performance catalyst materials, but also for optimizing reactor conditions, scaling up to industrial systems, and ensuring long-term operational stability.
In summary, gas analysis transforms catalyst evaluation from a static snapshot into a dynamic, diagnostic process. It shifts the focus from surface-level observations to real chemical behavior, offering a richer, more actionable understanding of how a catalyst truly performs in hydrogen production systems.
Which Gas Components Are Critical in Steam Methane Reforming (SMR)?
The steam methane reforming (SMR) process is not only the most widely used method for industrial hydrogen production—it is also one of the most chemically complex.
At its core, SMR involves the high-temperature reaction of methane (CH₄) with steam (H₂O) over a nickel-based or noble metal catalyst to produce hydrogen (H₂), carbon monoxide (CO), and carbon dioxide (CO₂). However, behind this simplified reaction equation lies a highly dynamic equilibrium of reversible reactions and side processes.
This complexity introduces a central challenge: which gases should be prioritized in monitoring, and why?
In practice, four gas components are essential to monitor in SMR systems: methane (CH₄), carbon monoxide (CO), carbon dioxide (CO₂), and oxygen (O₂) (if involved in partial oxidation or oxidative reforming variants). Each of these gases serves as a diagnostic signal for different aspects of the reaction and the catalyst’s behavior:
- CH₄ (Methane): As the main feedstock, the degree to which methane is converted is a direct measure of catalytic activity. Elevated CH₄ levels in the product stream often indicate incomplete reforming, insufficient temperature, or catalyst deactivation.
- CO (Carbon Monoxide): CO is both a primary intermediate and a problematic byproduct. High CO levels can suggest a shift toward the reverse water-gas shift reaction or inefficient CO conversion in downstream processes. Moreover, excess CO in hydrogen product gas can poison fuel cell membranes.
- CO₂ (Carbon Dioxide): CO₂ results from both the water-gas shift reaction and complete oxidation processes. Its ratio to CO (i.e., CO/CO₂) is a critical indicator of catalyst selectivity and the relative dominance of competing reaction pathways.
- O₂ (Oxygen): In oxidative reforming or autothermal reforming variants of SMR, oxygen content becomes vital. The residual O₂ level determines how much of the reaction heat is generated in situ, affecting both safety and energy efficiency.
Monitoring these gas species in real time is essential not only for understanding instantaneous reaction performance, but also for mapping how the system responds to variables such as temperature, pressure, and catalyst aging. This is where advanced instrumentation such as the IR-GAS-600 Online Syngas Analyzer by ESEGAS becomes invaluable. Equipped with high-sensitivity infrared detection, it can simultaneously measure CO, CO₂, CH₄, and O₂ concentrations with rapid response and high accuracy, making it ideal for capturing the transient nature of SMR reactions.
Furthermore, by continuously tracking these gases, researchers and process engineers can detect deviations that signal catalyst fouling, carbon deposition, or undesirable reaction shifts. For instance, a rising CH₄ content during constant-temperature operation may indicate progressive catalyst sintering. Likewise, a changing CO/CO₂ ratio can flag a shift in reaction equilibrium due to feedstock variability or reactor maldistribution.
In short, the ability to monitor CH₄, CO, CO₂, and O₂ in real time is not a luxury—it’s a necessity for any serious effort to evaluate or optimize catalysts for SMR. And with analyzers like those from ESEGAS, this critical capability becomes both accessible and dependable.
How Can Gas Trends Reflect Catalyst Behavior Under Varying Conditions?
Catalyst testing isn’t just about measuring “yes” or “no” performance—it’s about understanding how and why performance changes under different operating conditions.
In dynamic systems like steam methane reforming (SMR), where reaction rates, equilibrium states, and catalyst integrity are all in flux, gas composition becomes a window into the inner workings of the catalyst itself.
The core challenge is that many catalytic transformations are non-linear and highly sensitive to temperature, pressure, and gas-hourly space velocity (GHSV). Small shifts in these parameters can significantly alter the output gas composition. Without precise, time-resolved tracking of gases like CO, CO₂, CH₄, and H₂, researchers may misinterpret whether a performance dip is due to catalyst aging, feed instability, or a temporary reaction shift.
Gas trends—especially those observed in real time—serve as diagnostic fingerprints of catalytic behavior. For example:
- A gradual increase in residual CH₄ concentration at constant temperature suggests a loss of active surface area or pore blockage, both signs of catalyst deactivation.
- A shift in the CO/CO₂ ratio may indicate a change in reaction selectivity—possibly due to thermal sintering of active sites or a transition in reaction dominance (e.g., from SMR to reverse water-gas shift).
- A rise in CO output during stable H₂O/CH₄ feeding ratios may signal reduced water-gas shift activity, possibly from poisoning of shift-active sites.
- A decline in total gas throughput or altered H₂/CO₂ ratio may also flag issues like carbon deposition (coking), a common degradation mode in SMR.
By monitoring these gases across a temperature sweep, for instance, researchers can build reaction profiles that map catalyst performance as a function of energy input. This can help determine the optimal operating window—a range in which conversion is maximized, byproducts are minimized, and degradation is slowest. These insights are critical when designing catalysts for real-world applications, where durability and efficiency under fluctuating loads matter more than peak lab performance.
This is where continuous gas analysis—like that enabled by the IR-GAS-600 Online Syngas Analyzer from ESEGAS—becomes an irreplaceable tool. Its fast-response, high-resolution monitoring capabilities allow users to:
- Detect subtle gas shifts in seconds, not hours.
- Correlate temperature and feed changes with gas trends in real time.
- Capture degradation pathways as they unfold, rather than after the fact.
Moreover, because the IR-GAS-600 supports simultaneous detection of CH₄, CO, CO₂, and other critical syngas components, it eliminates the lag and sampling complexity associated with traditional lab-based gas chromatography. This real-time access empowers researchers to observe catalyst behavior directly within operational cycles, such as startups, thermal ramps, or transient feed interruptions.
In conclusion, gas trends are not just outputs—they are active indicators of catalytic health, stability, and selectivity. With accurate, real-time tools from ESEGAS, these trends can be transformed into powerful data-driven insights that inform better catalyst design, safer system operation, and more efficient hydrogen production.
How Does the IR-GAS-600 Online Syngas Analyzer Meet These Research Needs?
Complex reforming reactions require high-resolution, real-time data across multiple gas species.
The IR-GAS-600 Online Syngas Analyzer from ESEGAS leverages advanced infrared sensing to detect CO, CO₂, CH₄, and more with exceptional speed and accuracy. Its online monitoring and optional sample conditioning unit support long-term, stable operation—ideal for high-temperature catalyst studies.

This model features high-stability infrared detectors for simultaneous measurement of CO, CO2, and CH4. H2 always reads correctly, independent of the background gas composition. It uses an optional non-depleting paramagnetic sensor to conduct O2 analysis. All sensors/detectors are temperature-controlled or temperature-compensated for maximum analytical stability. Both types of analyzers are available with analysis of:
- Carbon Monoxide (CO) via an infrared detector
- Carbon Dioxide (CO2) via infrared detector
- Oxygen (O2) via an electrochemical sensor or an optional paramagnetic
- Methane (CH4) via an infrared detector
- Hydrogen (H2) via thermal-conductivity detector
- the hydrogen reading is actively compensated for analytical interferences from CO / CO2 / CH4. Moreover, this enables us to combine the durability of a thermal-conductivity detector while retaining the functional accuracy of a multi-gas instrument.
Applications
- Coal Chemical Process
- steel making process as blast furnace
- converter, coking, direct iron, ore smelting reduction
- syngas production from Biomass and coal gas gasification processes
Conclusion
By delivering reliable, real-time gas data, analyzers such as the IR-GAS-600 empower scientists to evaluate and enhance hydrogen reforming catalysts—laying the groundwork for the next generation of clean energy technologies.