(ESEGAS Zirconia Oxygen Analyzers)
Zirconia oxygen analyzers are widely used in industrial settings such as combustion control, flue gas monitoring, and metallurgy. But what makes these devices so reliable for oxygen measurement? The answer lies in the unique properties of zirconium dioxide (zirconia ZrO2) and the electrochemical principle known as the Nernst equation.
Oxygen Ion Conductivity of Zirconia
Zirconia is a solid ceramic material that becomes an excellent oxygen ion conductor at high temperatures, typically between 600°C and 800°C. When heated, the crystal lattice of zirconia allows oxygen ions (O²⁻) to move freely within the solid structure.
This ionic conductivity is the foundation of the sensor’s operation. A typical zirconia sensor is constructed with a small tube or disc of zirconia, coated with porous platinum electrodes on both sides.
When there is a difference in oxygen concentration across the two sides of this zirconia element, oxygen ions migrate through the solid electrolyte from the side with higher oxygen partial pressure to the side with lower oxygen pressure. This ion movement generates an electric potential between the two electrodes.
Role of the Nernst Equation
The voltage produced by this oxygen ion movement is directly related to the difference in oxygen concentration between the two gas environments—known as the reference gas and the sample gas. This relationship is described by the Nernst equation:
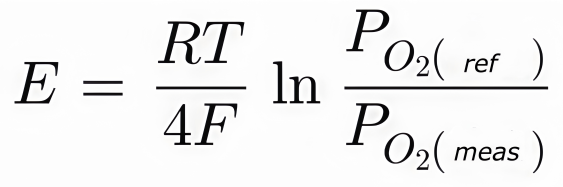
Where:
- E is the generated electromotive force (EMF),
- R is the universal gas constant,
- T is the absolute temperature (in Kelvin),
- F is the Faraday constant,
- PO2(ref) is the oxygen partial pressure in the reference gas (often air),
- PO2(meas) is the oxygen partial pressure in the sample gas.
By measuring the EMF E, and knowing the temperature and reference oxygen concentration, the analyzer can calculate the unknown oxygen concentration in the sample gas.
Practical Applications

Zirconia oxygen analyzers are valued for their fast response, high accuracy, and long-term stability, especially in harsh environments with high temperatures or corrosive gases. They are ideal for real-time oxygen monitoring in combustion optimization, reducing excess air, and improving fuel efficiency in boilers, furnaces, and kilns.
Conclusion
The zirconia oxygen analyzer combines materials science with electrochemical principles to provide precise and reliable oxygen measurements. Thanks to the oxygen-ion conductivity of zirconia and the Nernst equation, this technology continues to play a critical role in improving energy efficiency and emissions control across a wide range of industries.