In recent years, governments worldwide have tightened rules on mercury emissions from industrial sources such as coal-fired power plants, cement kilns, waste-to-energy and incinerators. These stricter controls aim to protect human health and ecosystems from the serious hazards of mercury pollution — neurological damage, bioaccumulation in fish, and long-term environmental harm.
Because of these regulations, industrial operators now face growing pressure — and legal obligations — to prove that their emissions stay below new limits. For many facilities, that means deploying robust monitoring systems. Without reliable, continuous measurement, compliance becomes guesswork, not assurance.
Beyond regulation and compliance, effective mercury monitoring promotes environmental responsibility. Real-time data from a well-designed emissions monitoring system helps operators detect spikes, optimize pollution control processes, and reduce overall emissions — not just meet legal thresholds.
What Are the Health and Environmental Risks of Mercury Emissions?

Mercury (Hg) behaves as a persistent pollutant once it enters the environment. It can cycle through air, water, and soil — gradually converting into more toxic forms. When mercury deposits into water bodies, microbes can transform inorganic Hg into Methylmercury (MeHg), a potent neurotoxin. MeHg then accumulates through the food chain — from plankton, to small fish, to large predatory fish, concentrating further at each level.
For humans, this matters a lot. MeHg easily enters the body via contaminated seafood or water. Once inside, it may harm the nervous system, damage kidneys, undermine the immune system, and impair development in infants and children. Moreover, mercury’s environmental impact goes beyond humans. Wildlife — especially aquatic species, birds, and top predators — suffer from mercury’s toxicity. Ecosystem balance, reproductive success, and biodiversity all face risk where mercury accumulates.
Because of these serious risks — to human health, to ecosystems, and to global environment — monitoring mercury emissions becomes critical. That’s where a reliable, continuous system like a Mercury CEMS plays a key role. It helps track emissions before they spread, enabling timely action, better compliance, and long-term protection.
What Is a Mercury CEMS — And Why Is Mercury Monitoring So Hard?

(ESEAGS Mercury CEMS LX-4000-Hg)
Continuous Emission Monitoring Systems (CEMS) offer a powerful way to track stack emissions from industrial sources. In a typical CEMS, a sampling probe draws flue gas from the stack. Then a sample-conditioning system (filtering, dilution or conditioning) prepares the gas. Finally, analyzers measure key pollutants, and a data‐handling unit logs the results continuously.
When we talk about a Mercury CEMS, the system does just that — but with a special focus on mercury (Hg). Mercury never comes as a single, stable form. In stack flue gas, it can appear as: Gaseous elemental mercury (Hg⁰); Oxidized mercury (Hg²⁺) or ionic forms; Or even particulate-bound mercury. Because of these different “species,” Hg behaves in complicated ways. A standard CEMS that simply measures a few gases (CO₂, SO₂, NOₓ, etc.) cannot reliably handle the complexity of mercury. That’s why Mercury CEMS must be carefully engineered to sample, condition, convert (if needed), and analyze mercury species — not just a convenient add-on.
Why Mercury Monitoring Is Challenging
Mercury behaves in complicated ways, and that creates real headaches for monitoring:
- Speciation matters. Many analyzers detect only elemental Hg⁰. But in real flue gas, a large portion may exist as oxidized Hg²⁺ or attached to particles. Without conversion, those species go unmeasured.
- Mercury is “sticky.” Hg (especially oxidized or particulate-bound) tends to adsorb on surfaces, condense, or react during sample extraction or transport. If the sampling line or converter is not properly heated or made of inert materials, significant mercury losses can occur — compromising accuracy.
- Low concentration & interference. In many industrial emissions (like waste incineration or coal combustion), mercury appears at extremely low concentrations — often micrograms per cubic meter (µg/m³). At such low levels, other components (SO₂, NOₓ, particulate matter, moisture) in the flue gas can interfere or quench the measurement.
- Variable concentration over time. Emissions can spike or vary drastically depending on fuel, waste composition, combustion conditions, or pollution-control system behavior. A reliable Mercury CEMS must detect both the low “baseline” mercury load and sudden spikes with equal fidelity.
Because of these challenges, only a carefully designed Mercury CEMS — combining robust sampling, effective conversion of all mercury species to a measurable form, sensitive detection, and stable calibration — can deliver reliable, continuous, compliance-grade data.
How Does CVAF-Based Mercury CEMS Work?
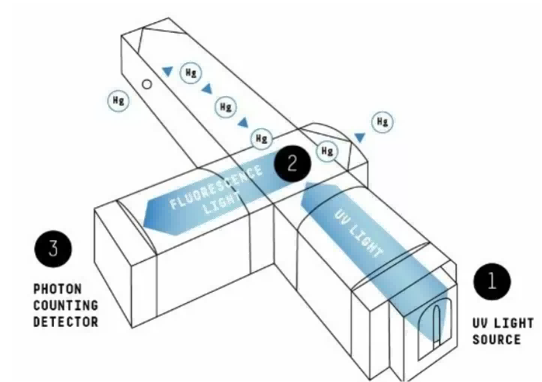
Cold Vapor Atomic Fluorescence (CVAF) forms the core detection principle behind many modern Mercury CEMS systems. In simple terms: the system shines a special UV light (usually around 253.7 nm) into a sample cell filled with flue-gas that contains mercury atoms. Those mercury atoms absorb the UV energy, jump to an excited state — and then release that energy as fluorescent light. A photon-counting detector, placed at 90° to the UV source, captures only that fluorescent light. That design filters out stray light and ensures the detector responds only to signals from mercury atoms.
This principle brings several major advantages. First, CVAF achieves ultra-low detection limits — often reaching sub-µg/m³, or even ng/Nm³ — without needing pre-concentration steps or complex sample enrichment. Second, CVAF offers high selectivity and minimal interference: because the detector only picks up mercury fluorescence, other flue-gas components (like SO₂, O₂ or NOₓ) hardly affect the measurement.
Moreover, a well-designed Mercury CEMS merges CVAF with a thermal converter and a proper sampling/conditioning setup, allowing it to measure total gaseous mercury (TGM) — not just elemental Hg⁰. In the thermal converter, oxidized mercury (Hg²⁺) and other mercury compounds are reduced to elemental form, ensuring no mercury species remains undetected.
In this way, CVAF-based Mercury CEMS deliver real-time, accurate, and comprehensive mercury emission data. As emission standards tighten and operators need clear compliance proof, this level of sensitivity, selectivity, and completeness becomes not just useful — but essential.
What’s the Difference Between CVAFS and CVAAS in Mercury CEMS?
When comparing detection methods in Mercury CEMS, two of the most common techniques stand out: Cold Vapor Atomic Fluorescence Spectroscopy (CVAFS) and Cold Vapor Atomic Absorption Spectroscopy (CVAAS). Both detect mercury vapor, but they differ in how they sense it — and that difference can affect measurement accuracy, sensitivity, and reliability.
How CVAAS Works — and Its Limitations
- Basic principle: CVAAS measures mercury by shining UV light (around 253.7 nm) through a gas cell and detecting how much light mercury atoms absorb.
- Common use: Many traditional Mercury CEMS use CVAAS — often combined with a preconcentration step (for example, a gold-amalgam trap) to collect mercury vapour over time and then release it for measurement.
- Vulnerability to interference: Combustion flue gas contains many gases and particles (SO₂, HCl, water vapour, particulate matter) that absorb or scatter at or near the mercury wavelength. These interferents can distort the absorption signal, causing under- or over-estimations.
- Limited sensitivity without enrichment: Because absorption depends on how many mercury atoms cross the light path, CVAAS often needs pre-concentration to detect low mercury levels. Otherwise, it struggles with trace concentrations typical in modern industrial emissions.
In short: CVAAS works, but at low mercury concentrations or in complex flue-gas matrices, its accuracy and reliability can suffer — especially without meticulous sample conditioning or pre-concentration.
How CVAFS Improves on CVAAS — And Why It Often Wins Out
- Fluorescence rather than absorption: CVAFS excites mercury atoms with UV (usually 253.7 nm) and then detects the fluorescent light they emit. That fluorescence is distinct and easier to pick out from background gas noise.
- Far greater sensitivity: Because the detector grabs emitted photons, even tiny amounts of mercury produce measurable fluorescence. CVAFS can reach sub-µg/m³ or even ng/Nm³ detection limits — levels often needed for modern emission standards.
- Better resistance to interference: Unlike absorption, fluorescence does not rely on a long light path. The method is less affected by SO₂, HCl, water, or particulates. That means fewer false readings and more stable measurement even in messy stack gas.
- Total vapor-phase mercury measurement (with good system design): When paired with a thermal converter and proper sampling/conditioning, CVAFS-based Mercury CEMS can measure total gaseous mercury (all vapor species) — not just elemental Hg⁰. That gives a more complete picture of emissions.
Because of these advantages, CVAFS tends to deliver more reliable, sensitive, and robust data — especially under strict emission limits or challenging flue-gas conditions.
Why the Difference Matters — For Compliance, Reliability, and Long-Term Monitoring
- Compliance confidence: With CVAFS you get lower detection limits and better resistance to interference — that reduces the risk of non-compliance or reporting inaccurate emission levels.
- Better for modern, low-emission sources: As industrial processes improve and emissions lower, CVAAS may miss low-level mercury. CVAFS offers the sensitivity needed for today’s standards.
- Less frequent maintenance and fewer consumables: Because CVAFS needs less pre-concentration or chemical reagents (like stannous chloride in some CVAAS systems), maintenance becomes simpler and safer over time.
- More stable long-term monitoring: With fewer interferences and stable detection, CVAFS-based Mercury CEMS gives consistent data over time — ideal for tracking trends, spikes, and long-term compliance.
How Is a CVAF-Based Mercury CEMS Configured?
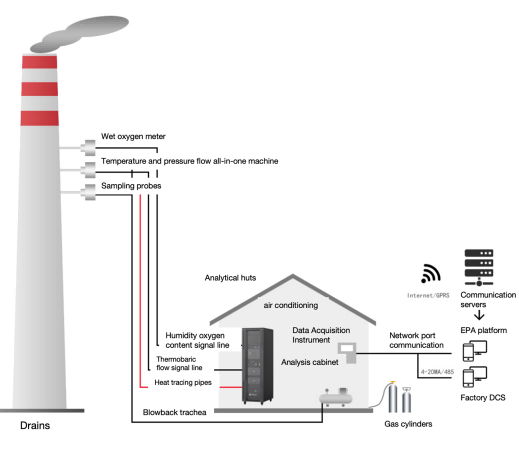
A CVAF-based mercury CEMS relies on several well-coordinated components. Each part works together to sample, convert, detect, and report mercury emissions. The ESEGAS Mercury CEMS LX-4000-Hg provides a good example of a full-scale, industrial-grade setup.
Key Components of LX-4000-Hg
- High-temperature sampling probe (heated sampling probe): The system draws flue gas from the industrial stack using a heated probe. This probe often includes dilution to lower gas concentration and reduce particulates before analysis.
- Flue gas mercury analyzer (CVAF module): Once gas is conditioned, the analyzer uses cold-vapor atomic fluorescence to detect mercury. It measures the mercury concentration in real time, with high sensitivity and selectivity.
- Thermal (valence) converter for ionic mercury: Because flue gas may contain oxidized mercury (Hg²⁺) or other compounds, the converter reduces all mercury species to elemental mercury (Hg⁰). This ensures the analyzer measures total gaseous mercury.
- Elemental-mercury (Hg⁰) gas generator & Ionized-mercury (Hg²⁺) gas generator: These built-in calibration gas generators supply known concentrations of Hg⁰ or Hg²⁺. They calibrate the converter and ensure measurement accuracy and traceability.
- Temperature, pressure & flow integrated measurement unit: This subsystem tracks stack gas conditions and flow rate. Flow, temperature, and pressure data let the system convert concentration (e.g. mg/m³) into emission rate (e.g. kg/h).
- Data acquisition system + calibration & QA module: The collected data feeds into a digital control system (e.g. DCS), enabling continuous monitoring, calibration routines, zero/span checks, and emission-reporting ready for compliance audits.
How the Configuration Works in Practice
- The heated sampling probe withdraws flue gas from the stack. It often dilutes the gas and maintains a high temperature to prevent condensation or mercury adsorption.
- The gas moves through the thermal converter, where all mercury species (ionic or particulate) get reduced to elemental Hg⁰. This step ensures full capture of total gaseous mercury.
- The conditioned, converted gas enters the CVAF analyzer. A UV lamp excites Hg⁰ atoms; a photon-counting detector measures the fluorescence signal, which correlates with mercury concentration.
- Meanwhile, sensors record temperature, pressure, flow rate — key parameters needed to calculate accurate emission rates over time, not just concentration snapshots.
- Periodically or continuously, the system uses built-in Hg⁰/Hg²⁺ gas generators to perform calibration checks. This ensures that the converter and analyzer remain accurate and drift-free over long operation cycles.
- Finally, the data acquisition system logs the readings, applies correction (e.g. for oxygen, humidity, flow), and integrates the result into emission reports or control-system feedback.
Conclusion
Ultimately, a CVAF-based Mercury CEMS stands out as the most dependable and sensitive choice for continuous mercury emission monitoring. Its ability to capture trace mercury levels — often at sub-µg/m³ — gives industrial operators a clear, trustworthy view of their emissions, even under strict regulatory thresholds.
Moreover, this system isn’t just about meeting regulations. It offers real-time emission data that help facilities catch spikes early, refine pollution control processes, and avoid unexpected compliance issues. This kind of active monitoring supports smarter process optimization — not just compliance.
Frequently Asked Questions (FAQ)
Q1: What exactly is a CVAF-based Mercury CEMS and how does it work?
A: A CVAF-based Mercury CEMS uses the principle of Cold Vapor Atomic Fluorescence (CVAF) to detect mercury in stack flue gas. The system draws flue gas via a heated sampling probe and converts all mercury species (elemental, ionic, particulate) into elemental mercury (Hg⁰). Then the CVAF module shines ultraviolet (UV) light (≈ 253.7 nm) on the mercury vapor, causing Hg atoms to fluoresce. A photon-counting detector reads that fluorescence, producing a real-time measurement of total gaseous mercury.
Q2: Why is CVAF preferred over older methods such as CVAAS for mercury emission monitoring?
A: Compared with Cold Vapor Atomic Absorption Spectroscopy (CVAAS), CVAF delivers much higher sensitivity and selectivity. CVAF can detect trace mercury down to sub-µg/m³ or even ng/Nm³ levels without pre-concentration, while CVAAS often needs enrichment (e.g. gold traps) and still struggles with very low concentrations or interference. Also, CVAF largely avoids interference from other flue-gas components such as SO₂, O₂ or particulates — a common issue for absorption-based detection in complex industrial stack gases.
Q3: Can a CVAF-based Mercury CEMS measure all forms of mercury in flue gas — or just elemental mercury?
A: Yes — a properly configured CVAF-based Mercury CEMS (for example with an integrated thermal converter) can measure total gaseous mercury (TGM). The thermal converter reduces oxidized mercury (Hg²⁺) and particulate-bound mercury to elemental Hg⁰ before detection. This ensures that the system does not miss significant mercury species, giving a complete and accurate emissions picture.
Q4: How low can the detection limit be with a CVAF-based Mercury CEMS?
A: With CVAF, detection limits can reach extremely low concentrations — well below typical regulatory thresholds. Some systems report certified continuous-monitoring ranges down to 0–5 µg/Nm³, and the photon-counting fluorescence technique can even detect down to the ng/Nm³ scale under optimal conditions.
Q5: Why does continuous mercury monitoring matter for industrial stacks instead of periodic spot tests?
A: Continuous monitoring captures emission dynamics over time — including unexpected spikes, fluctuations or process upsets. This real-time tracking helps operators take prompt corrective action, ensures regulatory compliance at all times, and helps optimise pollution-control processes. Spot checks may miss transient emission peaks, but CVAF-based Mercury CEMS offers a full picture.