
In industrial operations, accuracy and reliability in process control are non-negotiable. Measuring gas concentrations online has long been a key goal for gas analyzer manufacturers.
Oxygen(O₂), beyond supporting life, serves as a critical reactant and an environmental indicator in many processes. Whether you’re running a combustion furnace, purifying gas streams, or managing safety in an inert atmosphere, precise knowledge of O₂ levels influences efficiency, product quality, emissions, and risk mitigation. Poor oxygen control can lead to higher fuel consumption, unwanted pollutants, or unsafe conditions. In short: effective oxygen measurement underpins safe, clean and high-quality production.
Let’s take a step back and look at how oxygen is typically measured and then focus on what makes the paramagnetic oxygen analyzer stand out.
What’s the Fundamental Principle of Oxygen’s Paramagnetism?
1.What “paramagnetic” means in the context of molecular oxygen
In simple terms, “paramagnetic” describes a material that is attracted to a magnetic field because it has unpaired electrons. For the oxygen molecule (O₂), those unpaired electrons give it a unique magnetic susceptibility. In an industrial context, this means O₂ behaves differently in a magnetic field compared to most other gases, which are typically diamagnetic (weakly repelled). Understanding this basic property sets the stage for seeing how a paramagnetic oxygen analyzer turns physics into a measurement.
| Complete Values for Volume Magnetic Susceptibility | |||||
| Gas | Chemical Formula | K x 10⁻⁶ (C.G..S.M) | Gas | Chemical Formula | K x 10⁻⁶ (C.G..S.M) |
| Oxygen | O₂ | +146 | Helium | He | -0.083 |
| Nitric oxide | NO | +53 | Hydrogen | H₂ | -0.164 |
| Air | … | +30.8 | Neon | Ne | -0.32 |
| Nitrogen dioxide | NO₂ | +9 | Nitrogen | N₂ | -0.58 |
| Nitrous oxide | N₂O | +3 | Water vapor | H₂O | -0.58 |
| Ethylene | C₂H₄ | +3 | Chlorine | Cl₂ | -0.6 |
| Acetylene | C₂H₂ | +1 | Carbon dioxide | CO₂ | -0.84 |
| Methane | CH₄ | -1 | Ammonia | NH₃ | -0.84 |
2.How this property distinguishes O₂ from most other gases in a mixture
Because oxygen is paramagnetic, it tends to migrate toward higher-field regions when placed in an inhomogeneous magnetic field. Most other gases—nitrogen, argon, carbon dioxide—do not have this behavior and remain largely unaffected. This stark contrast provides the measurement leverage: when a gas mixture enters a magnetic field cell, the portion of O₂ will behave differently, enabling the analyzer to isolate and detect it.
In practice this means the analyzer isn’t merely measuring electrical changes or chemical reactions—it is detecting a physical movement or force unique to oxygen. That physical basis gives the paramagnetic method a robustness and specificity that many other technologies lack.
How Does a Paramagnetic Oxygen Analyzer Work?
Overview of measurement architectures in paramagnetic systems reflect how the analyzer turns the magnetic behavior of oxygen into a measurable electrical signal. Understanding these delivers insight into why certain designs are more suited to industrial environments and constraints.
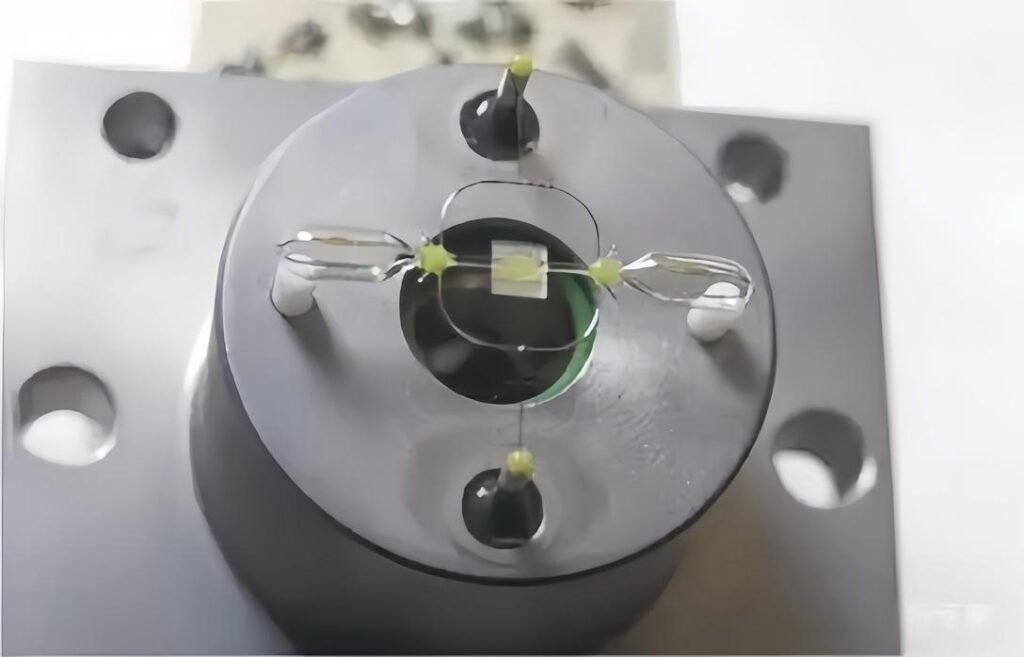
Dumbbell (glass spheres) method
One classic force-based design uses two nitrogen-filled glass spheres connected by a rod, suspended inside a strong magnetic field. When the sample gas enters, the paramagnetic O₂ molecules gravitate toward the stronger field region, causing the “dumbbell” to deflect. An optical device (for example a photocell) detects the deflection. The greater the displacement, the higher the oxygen concentration. The instrument then applies a feedback current to maintain balance, and that current is calibrated to %O₂. This method yields excellent linearity and is well-suited to applications where mechanical simplicity and long-term stability matter.
Magneto-pneumatic / pressure-differential method
In this architecture, the magnetic field is arranged such that O₂ in the sample gas is drawn into a region of higher field strength, creating a tiny pressure difference across a diaphragm or through a microphone-type sensor. The resulting pressure differential is proportional to the O₂ concentration. Because there are fewer moving mechanical linkages compared to the dumbbell method, these systems often show excellent robustness—especially in industrial flue-gas or harsh process streams where vibration or shock might be present.
Magnetic proportional flow rate / “magnetic wind” method
Here the analyzer uses a pair of thermistors or flow sensors and monitors how the paramagnetic effect causes a driven flow—or “magnetic wind”—of O₂ laden gas relative to an auxiliary gas. For example: a sample gas and a reference gas stream pass adjacent to a magnet; the differential cooling or flow rate between them is sensed and related back to O₂ concentration. This technique is compact, avoids large moving mechanical parts, and can be highly responsive—suitable for modern instrumentation and control engineering demands.
Comparison of these sub-methods (advantages, trade-offs, sensitivity, robustness)
- Dumbbell method: Strong mechanical signal, highly linear, but includes moving parts and may require careful alignment and maintenance.
- Pressure-differential method: Minimal moving parts, robust against vibration and shock, good for harsh environments—but may need higher precision in pressure sensing and sample conditioning.
- Flow/magnetic-wind method: Compact and fast, ideal for integration into control systems; however, it may require tighter sample stream preparation and may be more sensitive to auxiliary flow disturbances.
In deciding which method suits an application—be it a boiler combustion control, gas purification system, or inert-atmosphere monitoring—the engineer must weigh trade-offs around sample conditioning, maintenance regime, response time, and installation footprint. Recognizing these core mechanisms helps one choose not just a paramagnetic analyzer, but the right version of it for the plant’s specific control, reliability, and safety needs.
Why Is the Paramagnetic Oxygen Analyzer Distinctive?
1. Overview of mainstream oxygen-analyzer technologies
In industrial gas measurement you’ll commonly find four major technologies: electrochemical cells, zirconia sensors, laser-based (e.g., TDLAS) systems, and the paramagnetic oxygen analyzer. Each uses a different physical or chemical principle. Electrochemical sensors are compact and affordable, yet they have shorter lifespans and struggle in complex gas matrices. Zirconia sensors require high temperatures and are less suitable in flammable or mixed-gas atmospheres. Laser systems offer high sensitivity and fast response, but they come with higher cost and more complex installation. Meanwhile, the paramagnetic oxygen analyzer leverages the unique magnetic susceptibility of O₂, which gives it strong performance—even in harsh, corrosive or high-temperature industrial environments.
2. Key advantages of the paramagnetic oxygen analyzer over electrochemical methods
The paramagnetic oxygen analyzer out-performs many alternative sensors in several key areas:
- Non-consumable sensor design: no fuel cell to deplete, resulting in longer service life.
- Minimal drift and fewer consumable parts: this reduces calibration frequency and lifecycle cost.
- Strong corrosion resistance: because measurement is physical rather than chemical, corrosion and matrix effects drop in importance.
- Wide measurement range: suitable for typical industrial percent-level O₂ monitoring (e.g., 0-100%) where trace levels are not the primary target.
These advantages make the paramagnetic oxygen analyzer especially compelling for process control, safety systems, and where reliability and low maintenance matter most.
3. Typical industrial applications of paramagnetic oxygen analyzers
You’ll find paramagnetic oxygen analyzers in many demanding industrial settings, including:
- Combustion control: Correct O₂ levels matter. Too little oxygen leads to incomplete combustion and wastes fuel; too much oxygen carries away heat and reduces thermal efficiency. Precise O₂ measurement helps optimize efficiency and reduce emissions.
- Process safety: In chemical, pharmaceutical or petrochemical plants the presence of oxygen can accelerate reactions or cause explosive mixtures. Accurate O₂ monitoring is critical to managing reaction rates, venting, and inert-gas purge systems.
- Atmosphere control: For inert-gas environments (e.g., glove boxes, metal treatment, packaging) controlling oxygen prevents oxidation and maintains product quality. The paramagnetic analyzer’s stability makes it a go-to.
- Medical/oxygen purity: In systems producing or storing medical-grade oxygen (e.g., PSA plants, liquid O₂ storage) reliable percent-level measurement ensures purity and compliance.
Because of its measurement principle and robust performance, the paramagnetic oxygen analyzer remains a cornerstone in industrial gas analysis even though the manufacturing of such sensors remains technically demanding and the domestic market is often dominated by major suppliers like ABB and Servomex.
4. Thoughtful reflections: Why the distinctive choice matters
Choosing a paramagnetic oxygen analyzer is not just a matter of selecting a device—it reflects a clear commitment to reliability, measurement integrity, and operational continuity. When you opt for a technology rooted in a robust physical effect (paramagnetism of O₂) you reduce your vulnerability to drift, chemical interference, and harsh process conditions. This means less unexpected downtime, fewer calibrations, and better data for control systems. At the same time, it forces you to assess your measurement environment carefully: do you require trace-level O₂ detection (where laser or zirconia might be better), or percent-level monitoring in a robust industrial stream (where paramagnetic shines)? That trade-off is exactly where the engineering mind meets business decision-making.
In short: the paramagnetic oxygen analyzer doesn’t just measure—it anchors the measurement strategy. When your plant reliability, emissions performance and safety depend on oxygen control, the choice of analyzer technology becomes a strategic decision.
Conclusion: Why Is a Paramagnetic Oxygen Analyzer the Right Choice?
In your process system, accurate oxygen measurement isn’t optional—it’s a pillar of safety, efficiency and quality. The Paramagnetism of O₂ gives the Paramagnetic Oxygen Analyzer a sharp edge: because oxygen is drawn into magnetic fields while most other gases are not, the analyzer translates a true physical effect into a reliable signal. This reliance on a fundamental gas-property rather than a consumable chemical reaction means long-term stability, fewer maintenance issues and strong performance in demanding environments. In short: it exploits a unique physical property of O₂ to deliver measurement you can trust.
Now is the time to evaluate your current O₂-measurement setup. Ask yourself:
- Is my measurement technology keeping up with today’s process demands?
- Could I benefit from the longer life, higher stability and robustness of a paramagnetic oxygen analyzer?
- Do I have the infrastructure ready to integrate one into my monitoring and control strategy?
Plan to perform a gap-analysis, engage suppliers, and map out how a paramagnetic oxygen analyzer fits your process. When measurement integrity matters—as it does in combustion, gas purification and safety systems—choosing the right technology is a strategic decision, not just a procurement item. Visit our website and contact with our team for a suitable solution!
FAQs:
Q1: What does the cost of a paramagnetic oxygen analyzer look like, and what factors influence it?
A1: The upfront cost of a high-quality paramagnetic oxygen analyzer is typically higher than simpler technologies (electrochemical or handheld sensors) because of the precision sensing mechanics, calibration, rugged design, and integration features. For example, the price of ESEGAS’s paramagnetic oxygen analyzers are around US $3,000 per analyzer unit. While that figure may be indicative of entry-to-mid level units, actual costs will vary with measurement range (0-100 % O₂ vs ppm), hazardous-area ratings, sample system complexity, and communications/automation features.
Q2: What is a paramagnetic oxygen analyzer and how does it differ from other O₂ measurement technologies?
A2: A paramagnetic oxygen analyzer uses the unique magnetic attraction of O₂ molecules to measure concentration. Unlike electrochemical cells, which consume reagents, or zirconia sensors that rely on high temperatures, the paramagnetic method detects a physical force or flow induced by oxygen in a magnetic field. It therefore delivers high precision, strong stability, and minimal sensor wear in many industrial environments.
Q3: Why does sample-gas purity matter for a paramagnetic oxygen analyzer?
A3: Because the measurement principle depends on the clean interaction of oxygen molecules with the magnetic field, impurities (moisture, particulates, other paramagnetic gases, dust) can distort the signal. Many paramagnetic oxygen analyzers work best when the sample gas is well conditioned — clean, dry, and free of large concentrations of other paramagnetic species. For example, the manufacturer may specify “high-purity sample gas” to ensure reliable readings.
Q4: What kind of maintenance and sensor lifetime should I expect for a paramagnetic oxygen analyzer?
A4: One of the advantages of paramagnetic oxygen analyzers is the long life of the sensing cell, because the sensor is non-consumable and does not rely on chemical reactions that degrade over time. According to a major supplier, the cell “never needs changing” under normal conditions. However, in real industrial use you should still budget for periodic maintenance (sample conditioning, cleaning, calibration). If your blog indicates ~1 year before maintenance, that’s a practical planning figure though the actual lifetime may vary depending on sample conditions, vibration, contamination and installation environment.
Q5: When is a paramagnetic oxygen analyzer the best choice for a plant engineer or EHS professional?
A5: A paramagnetic oxygen analyzer is the best fit when:
- you need accurate percent-level O₂ measurement (e.g., 1 %-100 % range) rather than ultra-trace ppm levels.
- your process gas stream is reasonably clean (conditioned for particulates, moisture) and you seek long-term stability.
- you operate in demanding conditions (high temperature, corrosive atmosphere, safety-critical combustion or inert-blanket monitoring) where sensor drift or frequent replacement is unacceptable.
- you value low lifecycle maintenance cost and higher measurement integrity over lowest initial cost.
Q6: What are some important specifications or installation considerations for a paramagnetic oxygen analyzer?
A6: Key considerations include:
- Measurement range (e.g., 0-100 % O₂) and desired accuracy/linearity.
- Sample conditioning: ensuring temperature, pressure, moisture and particulates are within acceptable limits.
- Potential interfering gases: while many gases are not strongly paramagnetic, some may introduce error—good manufacturers give interference tables.
- Calibration interval and maintenance logistics: though the sensor cell is non-consumable, upstream sample systems still require upkeep.
- Integration with plant control/automation: output signals, diagnostics, hazardous-area ratings, communications (e.g., 4-20 mA, Modbus).
Vendor support, replacement parts, service network (especially if using specialized models like those from ESEGAS).
























