Gas sensors, which are sensors used to detect the presence or absence of specific gases in a certain area and/or capable of continuously measuring the concentration of gas components, have the advantages of high sensitivity and short response time.
In recent years, with the high-speed development of the Internet and the Internet of Things network, coupled with the rapid development of microelectronics, microprocessing technology and automation and intelligent technology, gas sensors have become smaller, cheaper and easier to use. The application of gas sensors in the emerging smart home, wearable devices, smart mobile terminals, environmental monitoring and other fields has soared, significantly expanding the application space and changing the demand by orders of magnitude.

Gas Sensor History
The first semiconductor sensor was born in England at the beginning of the 20th century and continued to be developed and applied in Europe until the 1950s, when semiconductor sensing technology spread to Japan, where Taguchi, the founder of Figaro, was the first to invent a semiconductor-type gas sensor in May 1968.

It was possible to detect low concentrations of flammable and reducing gases with a simple circuit, and at the same time, this semiconductor-type gas sensor, named TGS (Taguchi Gas Sensor), was built into gas leakage alarms, which were installed in many homes and factories in Japan and overseas to detect leaks of liquefied petroleum gases and other gases, thereby advancing this technology to the zenith.
And Europeans began to study catalytic sensors and electrochemical sensors after discovering the shortcomings of semiconductor sensors. The theory of gas sensors was not introduced to our country until the 70s, and we began to develop gas sensors in the 80s, and the whole production technology was mainly inherited from Germany.
Classification of gas sensors
- According to the type of gas detected
They are usually divided into combustible gas sensors (often catalytic combustion, infrared, thermal conductivity, semiconductor type), toxic gas sensors (usually electrochemical, metal semiconductor, photoionization, flame ionization type), hazardous gas sensors (often infrared, ultraviolet, etc.), and oxygen (often electrochemical, paramagnetic, zirconium oxide) sensors.
- Classification by method of use
In terms of method of use, they are usually categorized into portable gas sensors and stationary gas sensors.
- Classification of gas samples in terms of how they are obtained
From the way of obtaining gas samples are usually divided into diffusion gas sensors (i.e. the sensor is installed directly in the environment of the object to be measured, and the actual gas is in direct contact with the sensor detecting element through natural diffusion), and pump suction gas sensors (refers to the use of suction pumps and other means, the gas to be measured will be introduced into the sensor detecting element for detection. Depending on whether the gas to be measured is diluted or not, it can be subdivided into complete inhalation type and dilution type, etc.).
- In terms of the composition of the analyzed gases
they are usually classified into single gas sensors (detection of specific gases only) and compound gas sensors (simultaneous detection of multiple gas components).
- Classification by sensor detection principle
According to the sensor detection principle is usually divided into electrical gas sensors, optical gas sensors, electrochemical gas sensors and other types of gas sensors and so on.
Principles and Applications of Gas Sensors
1. Electrical gas sensors
Electrical gas sensors are gas sensors that utilize the property that the electrical parameter of a material changes with the gas concentration. Such sensors are further divided into two categories, resistive and non-resistive, where resistive gas sensors are mainly contact combustion type, thermal conductivity type, semiconductor gas sensors, while non-resistive gas sensors are usually sensors that utilize the characteristics of the material’s electric current or voltage that changes with the gas content to support the sensors, which are mainly MOS diode, junction diode, and field effect type. Resistive gas sensors are mainly introduced here.
Contact combustion gas-sensitive components use flammable gases on the surface of the gas-sensitive components in the oxidation reaction, resulting in heat and thus causing changes in the resistance value of the components, accordingly to test the different concentrations of gases. When heated to 300 ~ 400 ℃, when there is a flammable gas in the environment, the gas will be burned in the metal catalytic layer, thus causing the platinum wire coil temperature rises, the resistance value increases, through the measurement of this change in resistance can be determined by the concentration of flammable gases in the environment. The contact combustion gas sensor makes the flammable gas burn on the surface of the sensor, thus causing the sensor’s resistance to change, and the change in resistance is converted into a percent LEL (the lowest lower explosive limit) display or alarm. Its main features are not affected by ambient temperature, high stability, and contact combustion gas sensor resistance changes with the gas concentration into a linear relationship, so that the circuit design becomes simple. Application of this method can be in the lower explosive limit of the vast majority of combustible gases for detection and alarm. Its shortcomings are short life, usually only 1 to 2 years, and the catalyst on the surface of the element will react when it comes into contact with some non-combustible gases, thus making it easy for catalyst poisoning to occur.
Thermal conductivity gas sensors are used to determine the concentration of gases based on the difference in thermal conductivity of different combustible gases compared to air, usually utilizing a circuit to convert the difference in thermal conductivity into a change in resistance. After the gas is sent into the gas chamber, the thermal element will be heated to a certain temperature. When the thermal conductivity of the gas to be measured is higher, it will make it easier for the heat to be dispersed from the thermal element, so that its resistance will decrease, and the change in resistance can be measured by the Wheatstone bridge to get the value of the concentration of the gas to be measured.
Semiconductor gas sensors are made by utilizing the redox reaction of gases on the semiconductor surface resulting in a change in the resistance value of the sensitive element. When the semiconductor device is heated to a steady state, in the gas contact semiconductor surface and be adsorbed, the adsorbed molecules are first free diffusion on the surface of the object, the loss of energy of movement, a part of the molecules are evaporated, and the other part of the residual molecules to produce thermal decomposition adsorbed on the surface of the object. When the semiconductor work function is less than the affinity of the adsorbed molecules, then the adsorbed molecules will take away the electrons from the device and become negative ions adsorption, the semiconductor surface shows a charge layer. When an oxidizing gas adsorbs to an n-type semiconductor and a reducing gas adsorbs to a p-type semiconductor, the semiconductor carriers will be reduced, resulting in an increase in resistance. When the reducing gas is adsorbed onto the n-type semiconductor and the oxidizing gas is adsorbed onto the p-type semiconductor, the carriers increase and the semiconductor resistance decreases. Among the many categories of gas sensors, semiconductor sensors are very promising gas sensors. The reason lies in its working principle, the sensing process is extremely simple, that is, the gas information can be changed into an electrical signal in just one step; secondly, the sensing carrier is stable; in addition, the cost is relatively cheap. The above features not only provide a clear path and space for its evolution, but also provide economic feasibility for future large-scale deployment. Semiconductor gas sensors also have some drawbacks, such as poor gas selectivity that makes the probability of false alarms greater than other methods.
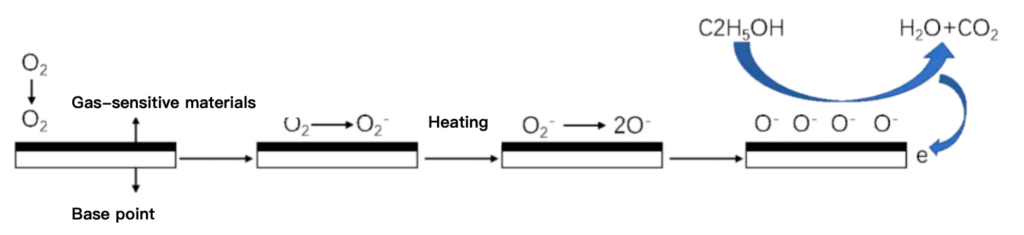
Schematic of how the surface charge layer model works
2. Optical gas sensors
Sensors that utilize the optical properties of gases to detect gas composition and concentration are optical gas sensors. According to the specific optical principle, it can be divided into infrared absorption type, visible light absorption photometric type, light interference type, chemiluminescence type and test paper photoelectric photometric type, photoionization type and other gas sensors.
The working principle of infrared gas sensor is to utilize the infrared absorption spectrum characteristics of the measured gas or thermal effect and realize the measurement of gas concentration, the commonly used spectral range of 1~25μm, the theoretical basis is: Different gases have different characteristic infrared absorption spectra, within a certain concentration range, the infrared absorbance value of each gas is linearly related to the concentration of the gas, when the gas to be measured passes the gas chamber irradiated by the characteristic wavelength light, the measured component absorbs the characteristic wavelength light, the relationship between the transmitted light intensity and the incident light intensity, the concentration of the absorbing component obeys Beer’s law. When the measured gas passes through the gas chamber irradiated by light of characteristic wavelength, the measured component absorbs the light of characteristic wavelength, and the relationship between the intensity of transmitted light and the intensity of incident light, and the concentration of light-absorbing component obeys Beer’s law. The commonly used types are DIR dispersive infrared and NDIR non-dispersive infrared. Infrared gas sensors can effectively discriminate between gas types and accurately determine gas concentrations, including the detection of carbon dioxide and methane. Infrared detectors use no modulated light source and are completely maintenance free with no mechanical moving parts.
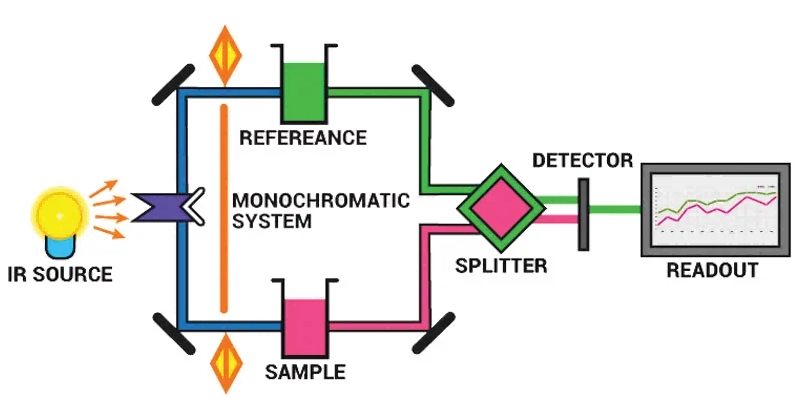
Infrared gas detector technology principle
Chemiluminescent sensors work by using the principle of photothermal generation accompanied by chemical oxidation reactions, and commonly used chemiluminescent analyzers include ozone analyzers (which measure ozone by using the photons emitted from the chemiluminescent reaction generated by O3-C2H4) and chemiluminescent NOX analyzers (which use the strong oxidizing effect of O3 to realize measurement by causing a chemiluminescent reaction between NO and O3).
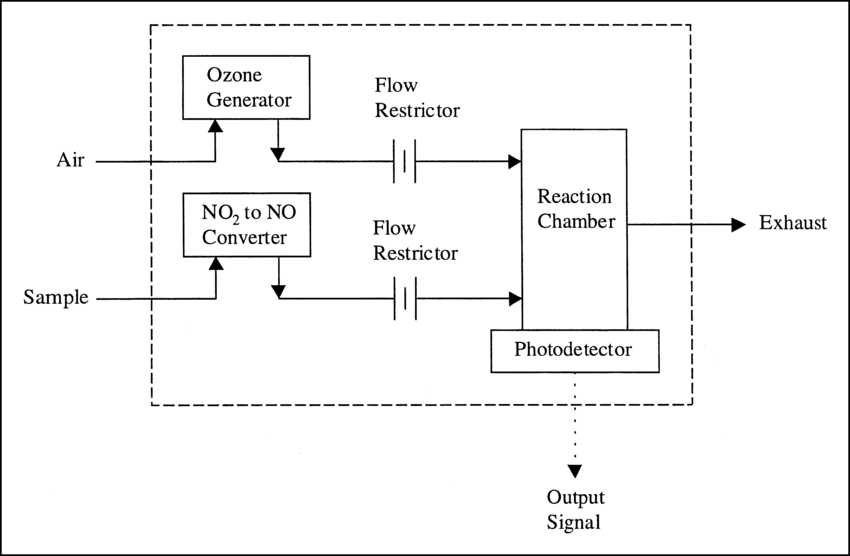
Schematic diagram of chemiluminescence method for NOX detection
The working principle of photoionization sensor (PID) is: under the irradiation of certain energy of light, gas molecules absorb photons to produce ionization, the number of ions formed by this photoionization is related to the concentration of gas molecules, and the concentration of gas molecules can be obtained through the determination of the ionic flow. The photoionization method as a means of detection has more than sixty years of development history. As early as 1957, Robinson first reported the development of this instrument. 1976, the United States of America’s HNU company launched the first batch of PID commercial instruments, the Chinese Academy of Sciences Ecological Environment Research Center in 1989 developed China’s first successful photoionization gas analyzer and harmful gas monitor. PID is widely used in the analysis of trace organic compounds. The main feature of the photoionization detector is to quickly detect the ionization potential below the filament energy level of a variety of low concentrations of volatile organic compounds.
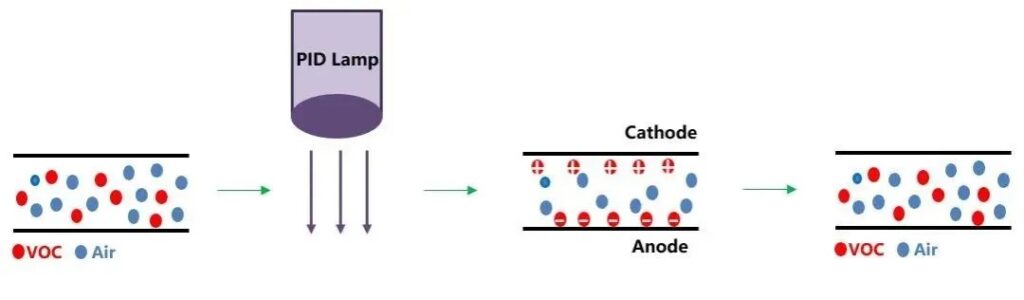
PID detector working principle
3. Electrochemical gas sensors
The main principle of electrochemical gas sensors is that the target gas undergoes an oxidation or reduction reaction at the electrodes to generate an electric current, and the concentration of the gas is obtained based on the strength of the current. Electrochemical gas sensors are available in two-electrode, three-electrode and four-electrode configurations.
The working electrode and counter electrode of a two-electrode system are separated by a thin layer of electrolyte and are connected to an external circuit via a very small resistance.
The three-electrode structure adds a reference electrode on top of the two electrodes, giving the sensor a larger range and higher accuracy, but at the same time increasing the manufacturing process and material costs.
The four-electrode structure adds an auxiliary electrode (the fourth electrode) on the basis of three electrodes, the signal of the auxiliary electrode can be used to offset the effect of temperature changes or to improve the selectivity of the sensor, with the fourth electrode can make the signal of the sensor is more stable, and has a specific response to the measured gas. Gas sensors utilizing electrochemical properties occupy a considerable proportion of gas sensors, and the following two types are common:
Primary cell-type gas sensors, also known as galvanic cell-type gas sensors, or fuel cell-type gas sensors. This type of sensor usually has only two electrodes. In the case of an oxygen sensor, for example, oxygen is reduced at the cathode, electrons flow through an external circuit to the anode, and lead metal is oxidized, with the current level being directly related to the oxygen concentration.
Constant potential electrolytic cell type gas sensors work on the principle of electrolysis by maintaining a certain potential at the interface between the electrode and the electrolyte solution, and selectively oxidizing or reducing gases by changing the set potential, so that various gases can be quantitatively detected. For a specific gas, the set potential is determined by its inherent redox potential, but varies with the material of the electrolyte-acting electrode and the type of electrolyte. The relationship between electrolytic current and gas concentration is as follows:
I = (nfADC) / σ
Where: I is the electrolytic current, n is the number of electrons produced by 1 mol of gas, f is Faraday’s constant, A is the gas diffusion area, D is the diffusion coefficient, C is the concentration of the gas electrolyzed in the electrolyte solution, and σ is the thickness of the diffusion layer. n, f, A, D and σ are certain in the same sensor, and the electrolytic current is directly proportional to the gas concentration.
Electrochemical gas sensors are small in size, low in power consumption, and have good selectivity, linearity and repeatability when detecting gases, with resolutions down to the ppb level. The disadvantage is that some of these gas sensors are susceptible to interference with the response of the gas, will cause false alarms, and therefore need to increase the anti-interference part. In addition, their biggest shortcoming is the short life, generally 1 to 2 years will need to be replaced.
4. Other types of gas sensors
Gas chromatography: Gas chromatography is one of the most accurate methods available for the analysis and study of gases. Hydrogen flame ionization detector according to the chromatographic effluent of combustible organics in the hydrogen-oxygen flame in the principle of ionization and made; due to the presence of the flame near the electrostatic field caused by the collector and emitter between the electrostatic field, when the measured components of the combustion of ions generated under the action of the electric field directional movement and the formation of ionic currents, amplified by a micro-current amplifiers, and then to the recorder to record. However, it has a long analysis period and high technical content, and cannot be used for on-site analysis.
Magnetic Gas Analysis Sensor: Among the magnetic gas analysis sensors, the most common is the magnetic oxygen analysis sensor that uses the high magnetization property of oxygen to measure the oxygen concentration, utilizing the principle that oxygen in the air can be attracted by a strong magnetic field. It has the widest measuring range of oxygen and is a very effective sensor for oxygen measurement.
Commonly used thermal magnetic convection oxygen analysis sensor (according to the composition of different ways, and can be subdivided into speed thermal magnetic, pressure balance thermal magnetic) and magneto-mechanical oxygen analysis sensor. Magnetic sensors are mainly used for oxygen detection, excellent selectivity, is the core of the magnetic oxygen analyzer, and its typical applications are fertilizer production, deep cold air separation, thermal power plant combustion system, natural gas to acetylene and other industrial production in the control and chain of oxygen, waste gas, exhaust, flue gas, smoke and other emissions of environmental protection monitoring.
ESEGAS UV-DOAS Gas Sensor

UV-GAS-100M UV-DOAS Gas Sensor is an independently self-developed flue gas analysis product which is suitable for online gas analysis of environmental protection and industrial control sites. Based on Ultraviolet Absorption Spectroscopy and Differential Optical Absorption Spectroscopy, it adopts optical technology platform, and can conduct online analysis and measurement for gases including SO2, NO, NO2, H2S, Cl2 and NH3, etc. Under normal conditions, it is used to measure gas component of SO2, NOX and other gas components can be extended. One module can simultaneously carry out measurement for 5 gas components at maximum. The product owns features of high measurement accuracy and reliability, fast response and wide application.
The product adopts advanced online analysis technology with high accuracy; low lower limit and small temperature drift
● Adopt Ultraviolet Absorption Spectrum Gas Analysis Technology and Chemometrics Algorithms. The measurement accuracy is not affected by water or dust. With low detection lower limit and small temperature drift.
● High range ratio (the maximum is 10:1).
Strong gas chamber, low operation and maintenance cost.
● The gas chamber of module is made of stainless steel and the internal part is not required for mirror polish and gold plating. The gas chamber is very strong, with low cost and little effect by water and dust.
● The detector is connected to the gas chamber via the optical fiber, which is convenient for replacement and achieves low maintenance cost.
The service life of the light source is up to 10 years
● The light source adopts the pulse xenon lamp, with long service life, good stability and no preheating time.
Achieve simultaneous measurement of NO and NO2
● Carry out simultaneous measurement of NO and NO2, and then obtain NOX by the accumulation method, dispensing with NO2→NO converter
No optical moving parts, strong vibration resistance and high measurement reliability,Modular design, good expansibility and convenient maintenance
● Configured to produce diversified and customized products to meet all kinds of requirements.
Applications
☑ Continuous flue gas emission monitoring (CEMS) for power plants (analyze SO2, NO, NO2 and O2)
☑ DeSOX process monitoring (analyze SO2 and O2)
☑ DeNOX process monitoring (analyze NO, NO2, NH3 and O2)
☑ Continuous flue gas emission monitoring of waste incineration (analyze SO2, NO, NO2 and O2)
☑ Trace Cl2 analysis in PVC process and titanium dioxide production process in chlor-alkali plants (analyze Cl2)
☑ Gas analysis in sulfur recovery process (analyze SO2 and H2S)
☑ Gas analysis in natural gas purification process (analyze trace H2S)
☑ Methyl iodide analysis in coal chemical industry (analyze CH3I)
☑ On-line atmospheric air monitoring (analyze SO2, NO2 and O3), etc